NAIROBI, Kenya (Kaab TV) – Indian authorities have banned the production and export of two addictive opioids after a BBC investigation exposed their role in fueling a public health crisis in West Africa.
In an official letter obtained by the BBC, India’s Drugs Controller General, Dr. Rajeev Singh Raghuvanshi, confirmed that approval for manufacturing and exporting these drugs had been revoked.
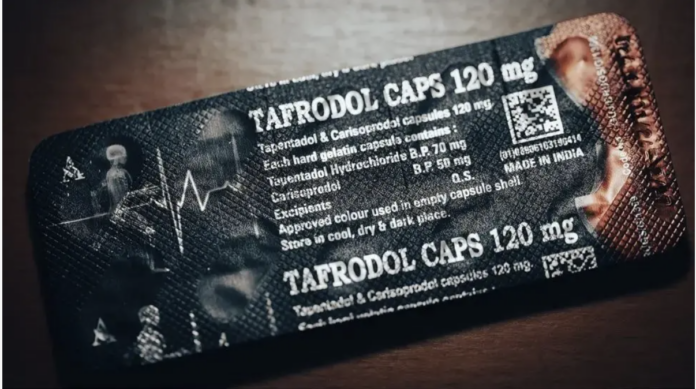
A BBC Eye investigation revealed that Indian pharmaceutical company Aveo had been illegally exporting a dangerous combination of tapentadol, a potent opioid, and carisoprodol, a highly addictive muscle relaxant, to countries including Ghana, Nigeria, and Côte d’Ivoire.
Following these findings, India’s Food and Drug Administration (FDA) raided Aveo’s Mumbai-based factory, seizing its entire stock.
Dr. Raghuvanshi’s circular, dated Friday, explicitly referenced the BBC’s findings as a key factor in the immediate ban on all tapentadol-carisoprodol combinations.
He cited concerns over the drugs’ high potential for abuse and their devastating impact on public health.
Tapentadol and Carisoprodol
Carisoprodol is banned in Europe due to its addictive properties but remains approved for short-term use—up to three weeks—in the United States.
Withdrawal symptoms can include anxiety, insomnia, and hallucinations.

The combination of tapentadol and carisoprodol is not licensed anywhere in the world, as it poses severe health risks, including respiratory failure, seizures, and potentially fatal overdoses.
Despite these dangers, the drugs are widely used as street narcotics in West Africa, where they are cheap and easily accessible.
Publicly available export data reveals that Aveo Pharmaceuticals, along with its sister company Westfin International, has shipped millions of these pills to Ghana and other West African nations.
The BBC World Service also discovered Aveo-branded pill packets being sold on the streets of Nigeria and in towns and cities across Côte d’Ivoire.
With a population of 225 million, Nigeria represents the largest market for these opioids.
According to the country’s National Bureau of Statistics, an estimated four million Nigerians misuse some form of opioid.
As part of its investigation, the BBC deployed an undercover operative posing as an African businessman seeking to supply opioids to Nigeria.
The operative infiltrated one of Aveo’s factories in India, capturing footage of Aveo director Vinod Sharma showcasing the same harmful products the BBC had traced to West Africa.
In the covert recording, the operative tells Sharma that the pills will be sold to Nigerian teenagers “who all love this product.” Sharma responds with a casual “OK” and explains that taking two or three pills at once allows users to “relax” and get “high.”
Later in the conversation, Sharma acknowledges, “This is very harmful for the health,” but dismisses the concerns, stating, “Nowadays, this is business.”
Neither Sharma nor Aveo Pharmaceuticals responded to the BBC’s request for comment following the publication of the initial investigation.
In a statement on Friday, India’s FDA confirmed that a sting operation led to the seizure of Aveo’s entire stock and the suspension of further production.
Legal action against the company is now underway.
The agency emphasized its commitment to cracking down on illegal pharmaceutical activities, declaring that it is “fully prepared” to take action against those who tarnish the country’s reputation.
Additionally, officials have been instructed to conduct further inspections to prevent the continued supply of these dangerous drugs.